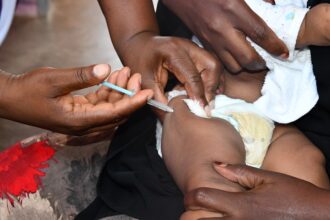
New vaccine campaign to eradicate polio kicks off
By Odhiambo David |odhisdavid59@gmail.com The fight against polio has received a boost…
Media science cafés on tuberculosis planned for November
By Christine Ochogo I christawine@gmail.com Improving awareness on gender-sensitive approaches to tuberculosis…
New malaria control initiative begins in Kenya as WHO gives nod to second vaccine to fight disease
By Odhiambo David | odhisdavid59@gmail.com A campaign which seeks to reduce malaria…
MESHA hosts regional media symposium on climate change, HIV and pandemics
By Joyce Chimbi I j.chimbi@gmail.com When COVID-19 took over life as people…
Tackling Nutritional Gap In Kilifi County
Juliet Anzazi is gently holding her 9-month-old child in her arms; today…
African countries urged to keep vigilance on COVID-19
Prof Jayne Byakika of WHO: She says that reduced public perception of…
Study: Immunisation programs unaffected by COVID-19
Caption: Dr Patrick Oyaro: Myths and misconceptions affect the uptake of vaccines…
Paying tribute to midwives around the world
A Nursing Mother from Thaba Phatšoa Ha Toboleloa ‘Mampho Raleting Liapeng Raliengoane:…
How efforts to counter resistance to Covid jabs have worked
Temperature check at a roadblock for COVID19. By Omboki Monayo | omboki2725@gmail.comAparticipation…
Urgent action needed to prevent premature deaths from HIV/AIDS
Ms Beatrice Anyiko has been a mentor mother since 2017. She sensitises…